sk life science xcopri
SK life science reserves the right to rescind revoke or amend this program at any time for any reason without notice. Get help talking to your doctor about whether FYCOMPA is right for you.

Xcopri Trademark Of Sk Biopharmaceuticals Co Ltd Registration Number 6110858 Serial Number 88485671 Justia Trademarks
One of SK Life Sciences trials for instance showed a 56 reduction from baseline in median seizure frequency compared to a 22 reduction among a placebo group over 12.
. See full safety PI boxed warning. Find what your patients may need. About XCOPRI cenobamate tablets CV Cenobamate is an anti-seizure medication ASM discovered and developed by SK Biopharmaceuticals and SK life science.
Learn More Get Started. SK Life Science Inc a subsidiary of SK Biopharmaceuticals Co Ltd an innovative global pharmaceutical company focused on developing treatments for central. Ad Learn about your options.
Ad EPIDIOLEX cannabidiol Is A Treatment Option Designed With Your Patients In Mind. Program managed by ConnectiveRx on behalf of SK life science. The Official Healthcare Professional Site For FDA-Approved EPIDIOLEX cannabidiol.
Assistance is Available for Eligible XCOPRI Patients. Still work to be done. Additionally SK life science will sponsor a product theatre session TV News Anchor Sarah Carlsons Treatment Journey with XCOPRI where physician Michael C.
The content on this site provides non-promotional scientific data on SK life science products. Ad Learn more about this treatment option on the official site for healthcare professionals. It was discovered and developed by SK Biopharmaceuticals and SK life science.
Tools resources and other information are available. We explore the complex mysteries of the brain to find. Access tools and support.
XCOPRI is an anti-epileptic drug AED for the treatment of partial-onset seizures in adults. The Official Healthcare Professional Site For FDA-Approved EPIDIOLEX cannabidiol. SK life science Presents Long-Term Data of XCOPRI cenobamate tablets CV in Adults with Partial-Onset Seizures at the American Epilepsy Society 2021 Annual Meeting.
He suffered from 24 seizures a year and this continued until he joined a clinical trial of SKs now approved epilepsy drug Xcopri which got him where is he is todayzero seizures. Recognising the current challenges faced by the US healthcare system with COVID-19 SK life science is committed to launching XCOPRI in a responsible way and is. As long as there are unmet needs in CNS we keep working.
In early 2019 SK. It is for informational purposes only and not intended as medical advice. Assistance is Available for Eligible XCOPRI Patients.
Ad Discover If XCOPRI May be a Treatment Option for You. Learn More Get Started. Ad Discover If XCOPRI May be a Treatment Option for You.
Because theresstill work to be done. Ad EPIDIOLEX cannabidiol Is A Treatment Option Designed With Your Patients In Mind.

About Us Sk Life Science Inc Sk Life Science Inc
Sk Biopharmaceuticals Signs 62 Million Deal To Export Anti Seizure Drug To Latin America Pharma 기사본문 Kbr

Xcopri Cenobamate Tablets Uses Dosage Side Effects Interactions Warning

Silver Branded Website For Consumers 2021 Mm M Awards Mm M Medical Marketing And Media

What Can I Expect When Taking Xcopri Cenobamate Tablets Cv Youtube
2 Ads For 2 Audiences Sk Life Targets Docs And Patients In Seizure Drug Campaign Fierce Pharma

Epilepsy Drug Xcopri Gets Schedule V Designation From Dea
Sk Biopharmaceutical Launches Xcopri In Us

40 Under 40 Stephanie Loiseau Sk Life Science 40 Under 40
Sk Life Science Plots 2020 Launch For Now Approved Seizure Drug Xcopri Gubba

2021 Pharma Choice Professional Website Bronze Winner Cdmp And Sk Life Science Inc Pm360

Sk Life Science Omnichannel Keynote Blackfin360 Innovation To Reality

Gary Ball Vp Sales Marketing Sk Life Science Inc Linkedin

Sk Life Science Receives Schedule V Designation From Dea For Xcopri Cenobamate Tablets
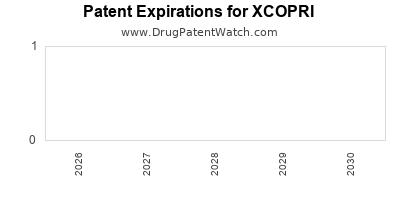
Xcopri Market Exclusivity Period Mep When Will The Patents On Xcopri Expire And When Will Generic Xcopri Be Available


